Release of unavailable phosphorus
After nitrogen, phosphorus (P) is the second element responsible for yields and crop quality. Its role in root development is well known. Optimization of phosphate fertilizer remains a key point. This optimization must be done according to agronomic, economic and ecological objectives, which are not always obvious to determine.
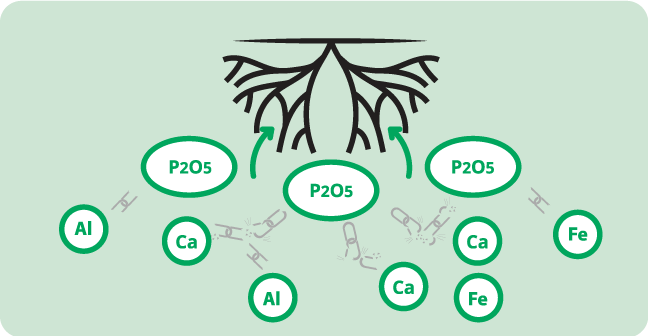
Failure to apply this element has multiplied in recent years. We must remain vigilant because even if several years without input may not lead to a significant drop in yields (in the case of rich and clayey soils), the day when the crops fall due to a phosphorus deficiency, the losses can be very significant. The farmer is then unable to resolve the problem quickly. The form that can be assimilated by the plant is P2O5. This form is mainly present in the grains, so the restitution or not of the straws has little effect on the quantity of phosphorus. The phosphate ions being very little mobile in the soil, the leaching of this element is very low. But on the other hand, it is for the roots of the plants to find this element which is difficult to assimilate because it has the particularity of getting stuck in the soil.
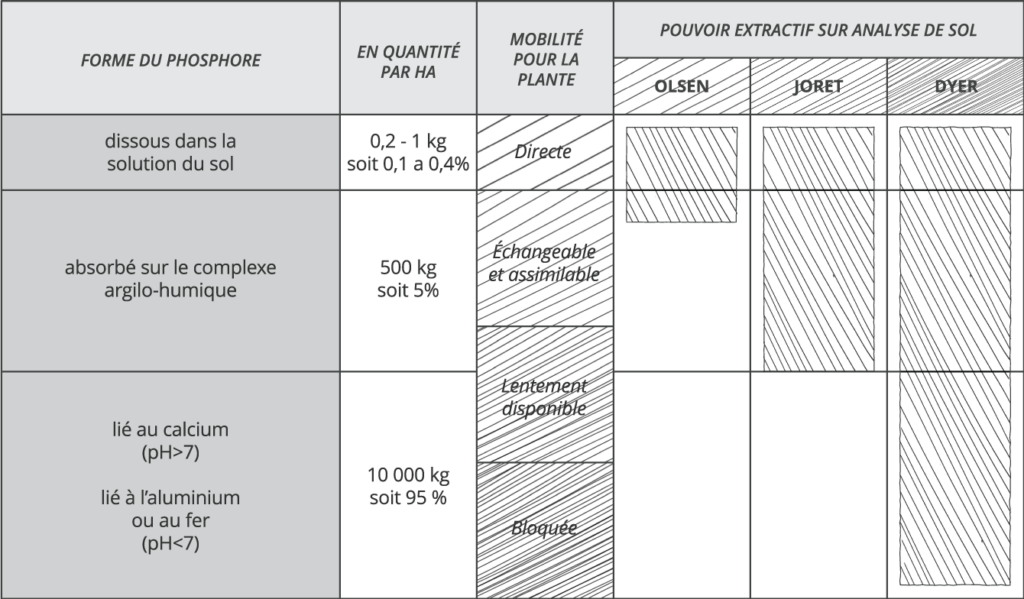
Due to its low propensity to leach, the average amount of total phosphorus in soils is generally high, up to 10,000 kg per hectare. Among this stock, about 500 kg are absorbed on the clay-humic complex and can become exchangeable and assimilable. Only 1 kg of phosphate ions per hectare is present in the soil solution at any given time. These ions represent the only directly assimilable food source for plants. The correct supply of phosphorus to the crop is therefore only determined by the soil’s ability to mineralize organic phosphorus and to desorb phosphorus from the solid phase in order to continuously supply the soil solution.
For this, two main parameters come into play: the pH and the temperature of the soil. Since the phosphates in the soil solution are anions (ions with a negative electric charge), they will naturally associate with cations (ions with a positive electric charge). In the case of acidic soils, it is mainly iron and aluminum (Fe2 + and Al2 +) which will bind to the phosphates and block it in the solid phase of the soil. This “fixation” phenomenon is very rapid since on average, 80 to 90% of the phosphate ions in solution in an acidic soil will be blocked within eight to ten days. In the case of calcareous soils, it is the calcium (Ca2 +), present in abundance, which will be the phosphate fixing agent and will create a blockage with it. This “fixation” phenomenon takes place slightly more slowly since on average 50% of the phosphate ions in solution in limestone soil will be fixed after 23 days.
In both cases, the binding of the phosphates with the cations will have the consequence of drastically reducing the availability of phosphorus for the crops. If, in the case of acidic soils, liming can remove some of the iron and aluminum phosphates to put them back into solution, the blocking phenomenon in calcareous soil is irreversible.
While pH has a primary effect on the durability of phosphorus in solution, temperature will play an important role in dissolving these phosphate ions, through its effect on microbial activity. In fact, the desorption of phosphorus from the solid phase of the soil and the mineralization of organic phosphorus are directly linked to the activity of the microbial flora. In fact, the desorption of phosphorus from the solid phase of the soil and the mineralization of organic phosphorus are directly linked to the activity of the microbial flora. On average, the bacterial activity of the soil will reach 30% of its potential for a temperature of 13 ° C, 43% at 16 ° C, 73% at 18 ° C and finally 100% for a soil temperature of 21 ° C.
In conclusion, the warmer and more neutral a soil, the more phosphorus it will make available to plants, and vice versa for cold soils with a pH moving away from neutrality.
Phosphate solubilizing bacteria are beneficial bacteria capable of solubilizing the mineral phosphorus. These bacteria such as Bacillus, Micrococcus, Pseudomonas, Flovobacterium or even Burkholderia are capable of releasing the mineral phosphorus blocked by the cations of the soil. This unblocking reaction takes place through the production of organic acids by bacteria: oxalic acid, lactic acid, malic acid, as well as by their enzymes called phytases. These enzymes will make it possible to release the phosphate ions thus making them available for the roots.
The life-killing sterilization temperature of the soil is 41 ° C. In France, a ground can rise up to 52 ° C in the sun while a covered soil does not exceed 28 ° C. Plowing should therefore be avoided, especially in August because the soil temperature will be high enough to kill the phosphate solubilizing bacteria.
It is also recommended to provide sufficient phosphorus available around the young roots, that is to say in the first ten centimeters of soil depth. It is then advisable to avoid the input before plowing which will dilute the phosphorus by thirty centimeters. Surface application after sowing is not recommended as the poorly mobile phosphorus will not descend sufficiently into the soil at root level. To limit blocking phenomena, it is therefore recommended to bring phosphorus as close as possible to the plant’s needs in space and time. The location of phosphorus next to the seed at the time of sowing is to date the best possible practice for enhancing this element.